Custom Tablet for Storz Shockwave Therapy Devices
Introduction
In a recent collaboration, Teguar joined forces with Storz, a medical technology manufacturer. Storz approached Teguar with a vision: to develop a tablet that would serve as the central command unit for their innovative shockwave therapy device. Unlike the provision of an off-the-shelf solution, Teguar set out to create a customized tablet, incorporating specialized medical certifications to meet the precise needs of the client.
Who is Storz?
Storz Medical is a renowned company specializing in the development and production of innovative medical equipment, with a particular focus on shockwave therapy devices. Founded in 1987 and headquartered in Switzerland, Storz Medical has established itself as a global leader in the field of extracorporeal shock wave therapy (ESWT).

Their expertise spans various medical disciplines, including urology, orthopedics, cardiology, and veterinary medicine. By continually pushing the boundaries of medical technology, Storz Medical aims to provide healthcare professionals with state-of-the-art tools that improve patient outcomes and enhance the overall quality of care. Their commitment to excellence and innovation has earned them a respected reputation within the medical community worldwide.
Project Details

The initial phase of the project highlighted the need for a hands-on approach, orchestrated by Teguar’s Custom Product Group. The primary requirement was a tablet capable of functioning as the command center for the shockwave therapy device. This device required not only standard operational capabilities and availability for many years, but also a ruggedness to endure the extensive and continuous shock waves inherent to its therapeutic functions. This necessitated a tablet that went beyond conventional specifications.
Solution Development
To meet these stringent requirements, the tablet underwent rigorous testing at an EMC (Electromagnetic Compatibility) facility based in Switzerland and Asia. Teguar’s product design specialist played a pivotal role by being physically present during the testing phases, ensuring real-time solutions to any challenges encountered. Here is one video of the tablet being tested:
The selected solution was Teguar’s TMT-Q7C80-10S tablet and desktop docking station. The tablet not only possessed the essential medical certifications but also demonstrated exceptional durability against constant shock wave exposure. These features made it the optimal choice for integration with the shockwave therapy device.
Customization extended beyond technical specifications to include aesthetic and branding elements. The tablet’s enclosure, docking station, and packaging were all custom designed to align with Storz’s branding directives. This approach was in line with Teguar’s motto, “Elegant Designs, Rugged Builds, Exceptional Service” emphasizing the creation of a tablet that was not only functional in a high-shock environment but also visually congruent with the client’s medical device. And, it was all possible due to Teguar’s solutioning with Storz.
Results
The outcome of this tailored development process was a device that met all expectations, thanks to the meticulous testing and proactive communication throughout the project. The custom tablet performed admirably under rigorous conditions, validating the extensive effort invested in its design and development. This tablet is compatible with the following Storz products:
- MASTERPULS MP50/100 »ultra«
- D-ACTOR 50/100 »ultra«
- DUOLITH SD1 T-Top F-SW »ultra«
- CELLACTOR SC1 T-Top C-ACTOR »ultra«
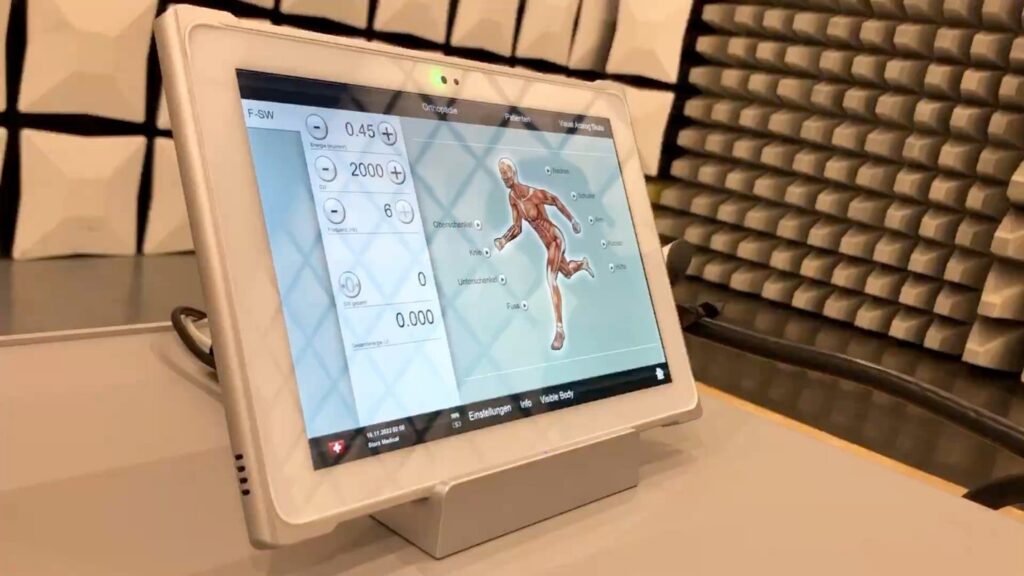
By going above and beyond to create a truly one-of-a-kind solution, Teguar succeeded in delivering precisely what the client envisioned.
Lessons Learned
Working with Storz Medical on this custom tablet project reinforced Teguar on several key lessons. Extensive and rigorous testing is crucial. By thoroughly evaluating the system at EMC and Regulatory labs while having engineering and product team members on-site to address challenges, Teguar ensured the tablet’s performance and reliability with the Storz shockwave solution as a whole.
Open and proactive communication among all stakeholders was essential to promptly address issues and meet the client’s specific needs. These experiences have strengthened Teguar’s approach to developing reliable and client-focused medical devices.
Conclusion
The development of the shockwave therapy tablet exemplifies Teguar’s commitment to working closely with Storz to meet specific needs and project requirements. This case study shows the value of tailored solutions in the medical technology sector, demonstrating that thorough testing, detailed customization, and strong client collaboration can lead to outstanding results.
Teguar’s shock wave tablet demonstrates how we work alongside clients to fill the exact need and requirements for their projects. If you have a need for a project or are interested in hearing more about the products we offer, you can contact a product specialist by calling +1 866 875 2148.
About the Authors:
Emily Vrettos, Teguar's Digital Marketing Coordinator, merges her creativity with marketing skills to craft engaging content. When she isn’t writing, she loves to read, cook lots of different cuisines, and travel home to her family in New England.Previous Article
Customized Off-the-Shelf Medical Computers Take on Unique Applications












